Through cooperative research of Professor Jae-Woong Jeong in our department and Michael Bruchas in University of Washington, their research team developed a wireless device for brain implantation which can control neural network precisely. The precise control is enabled by drug infusion and light emission to particular areas of the brain. This technology is expected to be applied not only to the development of new drugs which needs animal testing but also to the treatment of a brain disorder such as Parkinson’s.
The research, for which Raza Qazi (first author of the paper), Choong Yeon Kim, and Sang Hyuk Byun developed the device and in which neural science research team from University of Washington collaborated, was published in Nature Biomedical Engineering on 6th, August (The title of the paper: Wireless optofluidic brain probes for chronic neuropharmacology and photostimulation). By optogenetics and neuropharmacology, target neurons or neural circuits can be controlled without affecting other neural circuits. Because it has a much finer spatiotemporal resolution than conventional electrostimulation, this method is recently focused on the purpose of brain research and treatment of brain disorders.
However, current devices which are mostly used for brain research are some problems; damage to brain structure and their inability to control brain circuits precisely and be designed as one multifunctional probe. Also, there is a gap between soft brain structure and hard materials like silica and metal which are constitutive of the devices. With these reasons, those devices will aggravate infections so they are not appropriate to be implanted for a long time. The research team made tiny flexible probes by combining microfluidic probes and micro LEDs and fused the probes with small control circuits based on Bluetooth and replaceable drug cartridges. Thus, the team invented the neural-implantable device with a smartphone Bluetooth control of LED and drug infusion. Especially, the drug cartridges were designed to imitate LEGO so new cartridges can be connected to the device to provide drugs repetitively for a long time. Research team implanted the device on a target neural circuit of a mouse and combined cartridges with dopamine activator and inhibitor. Finally, the team succeeded in activating and inhibiting the behavior of the mouse by using the smartphone application.
In addition, the research team injected light-sensitive protein into the brain of a mouse to stimulate place preference. The team made the mouse prefer particular place by turning on the micro-LED when the mouse moved to the place. In contrast, the team successfully deleted place preference of the mouse using drugs. Professor Jae-Woong Jeong said, “nerve control using light and drug is more exquisite and this makes it possible to control the brain without side effects. The invented device needs the easy smartphone operation to control specific brain circuits using light and drugs repetitively and continually. Therefore, the device will be adapted to studies of brain functions and treatments of brain disorders.”
The research team is studying to improve the device to apply this technology to human by implanting the device in the human skull completely and perpetually.
Figure 1. Mouse with implanted control device Figure 2. Micro LED control by a smartphone app
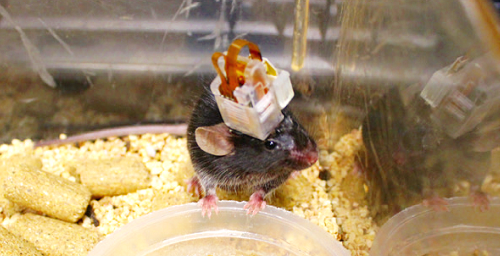

Figure 3. Developed wireless brain-implantable device
